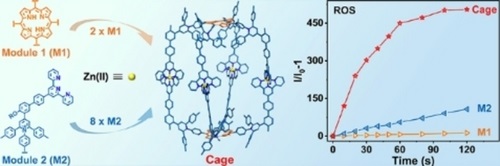
Photodynamic therapy (PDT) for cancer has attracted a wide attention due to its spatiotemporal selectivity and non-invasive features. Photosensitizers (PSs) are essential for PDT to harvest energy from light for generating reactive oxygen species (ROS). However, most of the PSs still limited to a few types of luminophore cores, including porphyrin and phthalocyanine, boron dipyrromethenes, xanthene, cyanine, Ru(II)/Ir(III) complexes and so on, which have been developed decades ago (Chem. Soc. Rev., 2021, 50, 4185). An alternative way of improving therapeutic efficacy of conventional PSs is loading them on the surface or inside of inorganic (J. Am. Chem. Soc., 2014, 136, 5647; Adv. Mater., 2020, 32, 2001862) or polymeric nanoparticles (Angew. Chem. Int. Ed., 2020, 59, 20582). Such a strategy, however, lacks structural precision for characterization and structure-property investigation.
In this study, we demonstrate an approach of synergistical incorporation of 10 luminophores into one metallo-supramolecule with high precision via facile pyrylium-aniline condensation of chromophore modules, and the subsequent coordination-driven self-assembly. Tetratopic building blocks are easy to be prepared with conventional PSs, e.g., porphyrin, zinc-porphyrin and tetraphenylethlene based on pyrylium-pyridinium salts chemistry for precisely constructing cage-like metallo-supramolecules. Specifically, the metallo-supramolecule with porphyrin core shows 6~12 folds higher ROS generation efficiency than porphyrin and rose bengal, which are the most widely used PSs for PDT. Moreover, this metallo-supramolecule displays strong emission for probing cells and specific interaction towards lysosome. With such features, subsequent cellular and living experiments further confirm the excellent photodynamic therapeutic efficacy, biocompatibility and biosafety of the constructed metallo-cage. See more details in our recent paper on Angew. Chem. Int. Ed. (Angew. Chem. Int. Ed., 2024, 63, e202400049).
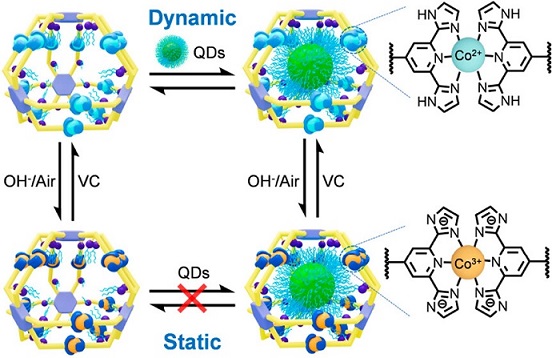
Self-assembly is a powerful bottom-up strategy for the construction of nanostructures with high precision, increasing complexity and designable functions. The precise self-assembly mainly rely on the dynamic and reversible interactions that connect the molecular components in a well-controlled manner. Among these diverse dynamic interactions, metal-ligand coordination has been extensively employed for the construction of sophisticated metallo-supramolecular architectures on account of its highly directional feature. However, the inherent reversibility of coordination often results in structural transformations or disassociation under different environments, such as temperature (Nat. Chem., 2017, 9, 903), concentration (J. Am. Chem. Soc. , 2016, 138, 12344), and pH (J. Am. Chem. Soc., 2019, 141, 815). The instabilities of metallo-supramolecules pose challenges to their characterization and applications, particularly under specific conditions such as complex in-vivo environments and catalytic reaction environments requiring high temperatures, strong acids/bases, and more. As such, achieving a delicate balance self-assembly reversibility and structural stability in metallo-supramolecular systems is emerging as one of the major challenges in the field.
To tackle this challenge, herein, we have developed a reliable in-situ stabilization strategy based on an acid/base-responsive ligand, 2,6-bis(imidazole-2-yl)pyridine (H2DAP), through in-situ conversion of dynamic <H2DAP-Co(II)-H2DAP> coordination junctions to static <DAP-Co(III)-DAP>. By utilizing the dynamic nature of <H2DAP-Co(II)-H2DAP>, a series of metallo-supramolecular cages with a diameter of ca. 3.8 nm is precisely constructed, and can undergo concentration-dependent structural interconversion and intermolecular ligand exchange in a highly dynamic manner. Through mild in-situ deprotonation/oxidation, the dynamic metallo-cages efficiently transform into their corresponding static states. These transformed architectures exhibit high stability to tolerate high temperatures, strong acids/bases, and metal chelators. Significantly, metallo-cages with distinct kinetic features and stability display different encapsulation and release behaviors by using nanoscale quantum dots (QDs) as guest molecules. This enables us to gain deeper insights into the encapsulation pathway and control the retention or release of large guests on demand through in-situ transformations. See more details in our recent paper on J. Am. Chem. Soc. (J. Am. Chem. Soc., 2023, 145, 18607).
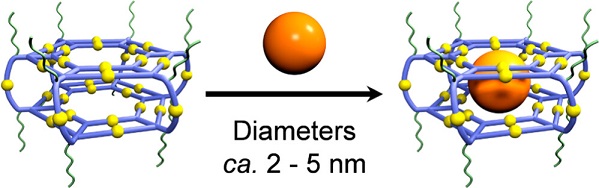
Synthetic nanospaces with desired cavities, including covalent and supramolecular hosts, have witnessed a rapid development ever since the discovery of crown ethers a half century ago, and the host-guest chemistry have remained an actively explored area (Science, 2009, 324, 1697; Science, 2019, 365, 159; Nature, 2019, 574, 511; Chem, 2020, 6, 3374). However, it is inconceivable that fullerenes have remained the largest guest substrate during the past three decades regardless if molecular or supramolecular containers are used as hosts (Nature, 1994, 368, 229; J. Am. Chem. Soc., 2017, 139, 11008), for the reason that giant assembled structures simply lack sufficient binding forces to trap larger substrates. As a result, most efforts on encapsulating nanocrystalline materials mainly rely on in situ growing nanoparticle guests inside hosts (Nat. Chem. 2010, 2, 25). However, owing to the harsh synthetic conditions required for most inorganic nanoparticles, use of host architecture to dictate energy and charge transfer between host and guest is still in an embryonic stage.
Optical properties of nanocrystalline materials within a confined space can be reshaped by their intimate interactions with the hosts. Particularly, acceptor-donor systems built around nanocrystalline materials such as semiconductor quantum dots (QDs) and metal nanoparticles display size and shape-dependent charge and energy transfer processes, which can be further regulated by the local environment surrounding the nanoparticles (Nat. Chem. 2012, 4, 310). In the field of supramolecular chemistry and nanotechnology, however, it remains a longstanding challenge to construct discrete nanospaces at molecular level precision to encapsulate nanoparticles and explore the photophysics of host-guest complexes.
In this work, we report an alternative yet widely applicable strategy for encapsulating inorganic nanoparticles in supramolecular cavities by leveraging the hydrophobic effect. A giant water-soluble supramolecular host system with large hydrophobic interior is constructed by introducing multiple polyethylene glycol (PEG) chains onto the outer surface of terpyridine-based supramolecular hexagonal prisms. Such a design of the host allows the selective encapsulation and confinement of hydrophobic QDs with diameters less than 5.0 nm in the supramolecular cavity, without the application of harsh conditions. Using a suite of optical techniques, we observe that the supramolecular hosts profoundly influence the dynamics of the QD guests by universally increasing the QDs’ relaxation rates via ultrafast electron and vibrational energy transfers. The hosts both inject photoexcited charges into the QDs, and increase the QDs’ ability to dissipate energy to its environment. See more details in our recent paper on J. Am. Chem. Soc. (J. Am. Chem. Soc., 2023, 145, 5191).
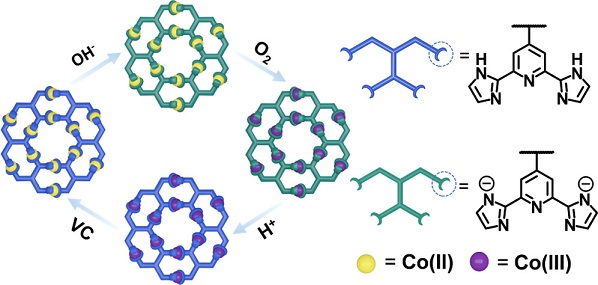
In nature, the interconversions of biomacromolecules among their different states play a crucial role in biological systems. These interconversions dominate a vast range of living processes at the molecular level, including enzyme catalysis, gene replication, nerve impulse, and magnetic responsiveness. Among all state-switchable bio-macromolecules, the heme proteins, e.g., hemoglobin and myoglobin, are representative examples. With very limited types of bio-macromolecular backbones, nature demonstrates that the manipulation of interconversions among different states in one system is a powerful approach to meeting diverse challenges in chemistry.
Therefore, it is of particular interest and importance to explore artificial metallo-supramolecular systems that are capable of switching their states reversibly in response to external stimuli. To date, many efforts have been made on constructing ensembles with interconvertible states (Nature, 1995, 374, 790; Angew. Chem. Int. Ed., 2016, 55, 445; Nature, 2018, 560, 65). However, only few examples can fulfill multiple states with distinct properties and functions without losing structural integrity, which are reminiscent of the diverse states of heme proteins. We reason that the underlying challenges mainly center on the following aspects: exploring a proper way of introducing stimuli-responsive functional groups onto the backbones of metallo-supramolecules; establishing a highly efficient approach for achieving complete interconversions among different states; and finding mild yet sufficient stimuli to prevent undesired side products during states transformation.
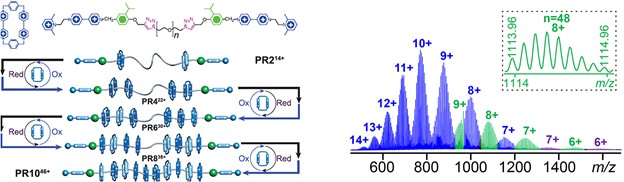
In the present work, we demonstrate an alternative approach of reversibly manipulating four states of metallo-supramolecular constructs under mild conditions, viz., acid-base and redox processes. The stimuli-responsiveness of the assembled supramolecular hexagonal wreaths mainly relies on the incorporation of a pH-responsive tridentate ligand 2,6-di(1H-imidazole-2-yl)pyridine (H2DAP) into the tetratopic building block. This is the first time for employing H2DAP containing multitopic building block in coordination-driven self-assembly, and three isostructural hexagonal wreaths with Fe(II), Co(II) and Ni(II) have been achieved. See more details in our recent paper on J. Am. Chem. Soc. (J. Am. Chem. Soc., 2023, 145, 3131).
Biological systems employ non-equilibrium self-assembly to create ordered nanoarchitectures with sophisticated functions. Inspired by nature, chemists have endeavored to construct artificial non-equilibrium assemblies over the past decades. In principle, the control over the assembly rates is of key importance for the assembly dynamics, kinetics and structures. In this context, many efforts have been devoted to tuning the self-assembly via optimizing the conditions or constantly supplying energy (Science, 2006, 313, 80; Science, 2011, 331, 1590; Science, 2015, 349, 1075; Nat. Nanotechnol., 2018, 13, 882; Nature, 2021, 594, 529). However, even slight fluctuations of conditions or external supply can substantially vary the assembly process, and therefore circumscribes the further development of artificial non-equilibrium systems.
Among many self-assembly approaches, coordination-driven self-assembly is regarded as one of the promising strategies to enable artificial non-equilibrium nanoassemblies owing to its excellent directionality and controllability. However, the majority of pioneering studies focused on the final assembled nanostructures under thermodynamic control (Nature, 1999, 398, 796; Science, 2006, 312, 1782; Nature, 2016, 540, 563; Science, 2010, 328, 1144; Nature, 2019, 574, 511), due to the rapid dynamics of assembly and lack of characterization at the comparable time scale. If the coordination process can be slowed down, we should have a chance to investigate the evolution of the transient intermediates under kinetic control.
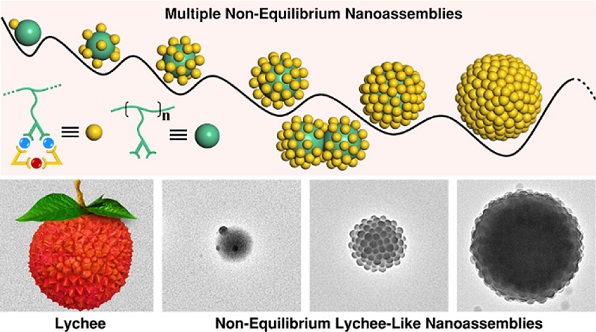
Given the self-assembly of side groups is confined by the polypeptide backbones in proteins (Science, 2012, 338, 1042; Nature, 2012, 491, 222) and the frustrated arrangement of side chains in grafting polymers (Chem. Soc. Rev., 2013, 42, 2075), we envision that the imposition of local constraints by spatial confinement should allow us to manipulate the assembly process and rate. As such, we design a series of polymers with different side groups for further coordination with shape-complementary components in this study. The polymer chain can impose a significant spatial confinement on the side groups, thereby frustrating the coordination-driven self-assembly to form supramolecular triangles. This frustrated behavior efficiently slows down the self-assembly kinetics and allows us to successfully observe and study non-equilibrium lychee-like nanostructures that are otherwise inaccessible in conventional coordination-driven self-assembly. See more details in our recent paper on J. Am. Chem. Soc. (J. Am. Chem. Soc., 2022, 144, 22651).
Molecular geometry represents one of the most important structural features. Nature employs highly efficient and precise approaches to construct a wide array of substances with distinct geometries and diverse functions. For instance, biosystems generate various types of polysaccharides through manipulating the connectivity between D-glucose, including amyloses, celluloses, cyclodextrins, and amylopectin/glycogen with helical, linearly extended, macrocyclic, and branched structures, respectively. These macromolecules with different geometries but similar chemical composition display specific (bio-)functions.
To reach the diversity and functionality of natural substances, increasing attentions have been concentrated on precise synthesis of macrocycles, helical polymers or foldamers, ladder polymers, etc. To facilitate the design and control of the desired configurations, molecular building blocks are usually designed with rigid backbone for ensuring specific shape, e.g., “V” convergent shape for constructing foldamers and “X” or “H” shape for ladder polymers. However, it still remains a formidable challenge to construct all these types of macromolecules based on the same building blocks or repeating units, perhaps owing to the lack of corresponding synthetic approaches for precisely manipulating the connectivity between monomers and feasible characterization techniques for visualizing macromolecules at single molecule level.
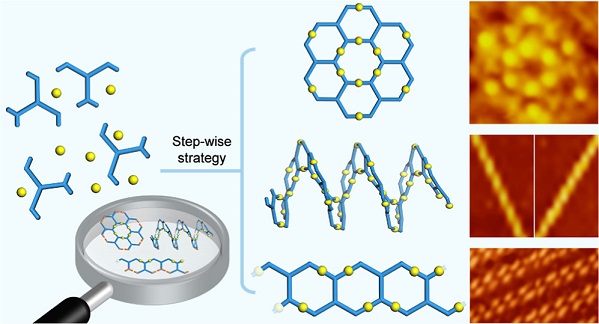
In this work, we combined the geometric features of “V”- and “H”-shaped building blocks to design and synthesize a series of tetratopic monomers with chevron stripe shape. By applying stepwise platinum-acetylide polymerization, we precisely manipulated the connectivity between the multi-topic monomers and thus successfully prepared four types of metallo-macromolecules, including concentric ring, helicoid polymer, ladder polymer, and cross-linked polymer. Furthermore, single-walled carbon nanotubes (SWCNTs) are selected as the guest to investigate the host-guest chemistry of these metallomacromolecules, in which helicoid polymer shows high efficiency in wrapping SWCNTs with geometry-dependent selectivity. To the best of our knowledge, we are, for the first time, able to construct different molecular geometries with identical repeating units in the context of metallomacromolecules. See more details in our recent paper on J. Am. Chem. Soc. (J. Am. Chem. Soc., 2022, 144, 16559).
Metallo-supramolecular cages have been of continuing interest to many research fields, particularly, chiral cages are gaining increasing attention because of their potential applications in chiral recognition, chiral separation and catalysis. In synthetic supramolecular chemistry, two main approaches have been employed to construct chiral cages: use of chiral and achiral precursors. The former approach is mostly used for generating chiral metallo-supramolecular cages. Although the latter one is a highly desirable approach without using any chiral source, racemization might be inevitable. Therefore, the achiral approach constructing chiral metallo-supramolecular cages still remains as a formidable challenge in the field of coordination-driven self-assembly.
Spontaneous resolution is the simplest way to obtain enantiomers from the racemic mixtures. However, only approximately 5-10% of racemic molecules are able to form conglomerates with spontaneous resolution. Most examples with spontaneous resolution were centered on small molecules. To date, only very few metallo-supramolecules assembled by achiral ligands were able to form conglomerates with chiral self-sorting behavior during crystallization (Angew. Chem. Int. Ed. Engl., 1993, 32, 703).
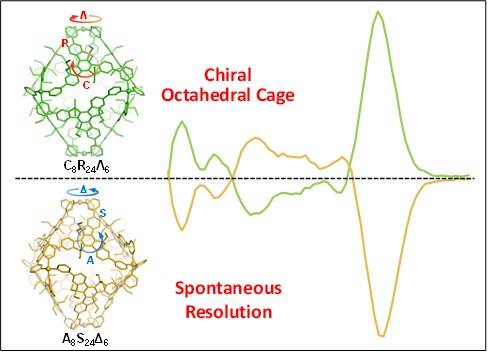
In this work, we construct metallo-supramolecular octahedral cages by a face-rotating strategy using a trispyridine ligand with C3h truxene core as the prochiral ligand. Racemate and conglomerate single crystals were obtained depending on the use of metal sources, i.e., Cu(BF4)2 yielded racemate crystals, while Cu(ClO4)2 and Cu(OTf)2 afforded conglomerate ones. In all cases, only homochiral cages were observed with three different types of chirality, including planar chirality from clockwise and anticlockwise arrangement of truxene core at the octahedral faces, the axial chirality from the S- and R- twisting between Py and truxene moieties at the edges, and the propeller chirality from Λ and Δ arrangement of Py around Cu(II) ion at the vertices. Remarkably, enantiopure cages were obtained by manually separating the conglomerate crystals without using any chiral auxiliary reagents or chiral HPLC separation method. The three types of chirality in solution state were further determined by CD spectroscopy and theoretical calculations, to show high stability with low racemization rate. For the first time, we are able to combine three types of chirality into a metallo-supramolecular cage with unambiguous identification of each one. See more details in our recent paper on Angew. Chem. Int. Ed. (Angew. Chem. Int. Ed., 2022, 61, e202203099).
Helical packing is ubiquitous in the natural world. It is seen with leaves, florets, and bracts of plants, and is typically referred to as parastichy in botany. Complex helical packing arrangements have fascinated artists and scientists for centuries. Different parastichy patterns, quantitatively described by the Fibonacci sequence, are well-established and used commonly to classify biological structures (Science, 1973, 181, 705). Moreover, the study of natural parastichies has revealed intriguing connections between number theory and packing theory (Science, 1977, 196, 270). However, natural parastichies have proved difficult to reproduce using simple systems, and non-natural parastichies are all but unknown.
In 1611, Johannes Kepler proposed that the densest packing for spheres would be achieved within a face-centered-cubic (FCC) arrangement and its stacking variants. This proposal was rigorously proved only recently by Hales (Ann. Math., 2005, 162, 1065). In the ensuing years considerable progress was made in the theory and numerical study of dense packings involving polyhedra (Nature, 2009, 460, 876; Nature, 2009, 462, 773; Science, 2012, 337, 453) and their connections to the self-assembly of polyhedral nanoparticles into ordered superstructures (Nat. Mater., 2012, 11, 13; Science, 2014, 345, 1149; Science, 2017, 355, 931). Nevertheless, since the report of the Boerdijk–Coxeter helix in 1952, very few helical packing structures based on either the Platonic or Archimedean solids have been reported (Philips. Res. Rep., 1952, 7, 303; Nature, 2009, 462, 773).
In this work, we have now found that by constructing a giant supramolecular truncated cuboctahedron (TCO, we are able to stabilize a new double helical form. This arrangement constitutes a new parastichy pattern that has not been seen previously either in nature or in the laboratory. We wish the excitement associated with this discovery and the aesthetic appeal that cuts across a wide range of disciplines from botany to number theory, and from Euclidean geometry to solid state chemistry will make this work tremendously broad in its appeal. See more details in our recent paper on J. Am. Chem. Soc. (J. Am. Chem. Soc., 2021, 143, 5829). This study was also highlighted by C&En entitled Self-Assembling Twisted Nanowires.


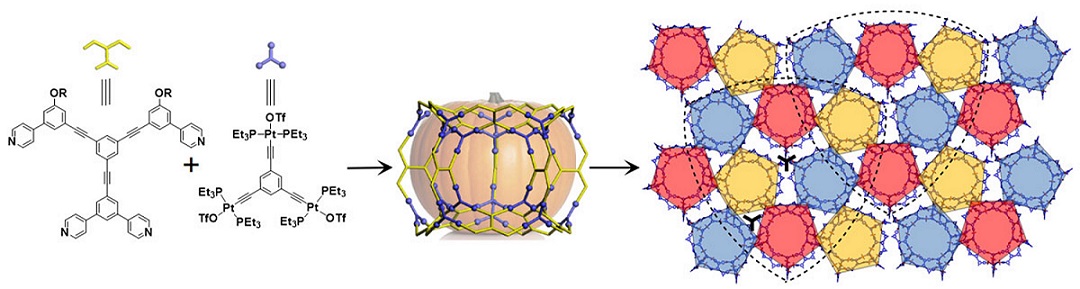
Pentagons are ubiquitous in the natural world and widespread in both art and architecture (Science, 2007, 315, 1106). However, a regular pentagon cannot completely fill the Euclidean plane (R2) due to its five-fold rotational symmetry. As a result, figuring out how to pack regular pentagons in R2 becomes a fascinating mathematical problem. It is, for instance, the 18th of Hilbert’s famous 23 problems (Bull. Amer. Math. Soc., 1902, 8). So far, only pentagon packings with global 2-, 4-, and 5-fold rotational symmetry have been achieved. One of the most famous examples is the Penrose pattern with 5-fold symmetry (Math. Intell., 1979, 2, 32). This mathematical prediction was subsequently realized at the atomic level with the discovery of quasi-crystals (Phys. Rev. Lett., 1984, 53, 1951; Nature, 1985, 316, 50). The seminal nature of this latter accomplishment is now widely appreciated. However, it is also an inspiration and leads one to ask whether it is possible to construct pentagon packings missing from the list of predicted possibilities, specifically those with global 3-fold rotational symmetry?
As detailed in this study, the answer is yes. Specifically, by creating giant “pumpkin-shaped” metallo-supramolecular cages (among the largest ever reported; cf. Science, 2010, 328, 1144; Nature, 2016, 540, 563) and allowing them to self-associate, one obtains crystalline superstructures with global 3-fold rotational symmetry. These constructs, which define an aesthetically pleasing “overlapping shield” arrangement, are characterized by an ultra-low, yet thermodynamically stable, pentagon packing (fraction of space covered by the polygons, as reflected in φ ≈ 0.576). This stands in sharp contrast to the commonly observed dense crystalline packing seen for pentagons (i.e., φ ≈ 0.921, Discrete Comput. Geom., 1990, 5, 389; Angew. Chem. Int. Ed., 2011, 50, 10612). See more details in our recent paper on Angew. Chem. Int. Ed. (Angew. Chem. Int. Ed., 2021, 60, 1298).
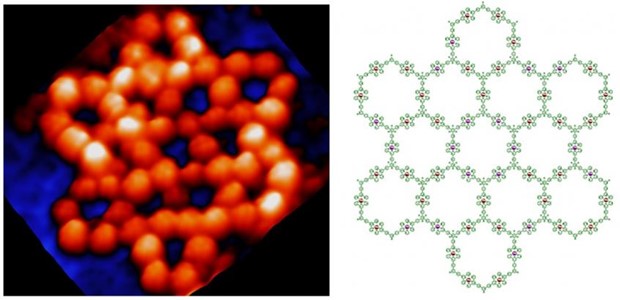
In the field of supramolecular chemistry, supramolecular chemists used metal coordination to construct a wide array of 2D and 3D structures with precise shapes and sizes, as well as tunable functions that can loosely model the features of structurally defined protein systems. Recent advances in structural biology have revealed the ubiquity of structural disorder or polymorphism in protein complexes. Systems with structural ambiguity or multiplicity are termed as fuzzy protein complexes, and are critical for a range of biological functions. However, such fuzzy structures have eluded effective mimicry using synthetic constructs. This is not for lack of desire, or even for effort. In present study, we combined folding and self-assembly, dynamic inputs used extensively by nature, to construct a giant fuzzy metallo-supramacromolecular hexagonal grid possessing both intrinsically ordered and disordered domains. With diameter >20 nm and molecular weight >65 kDa, this giant 2D supramolecular architecture is the largest one among all the published 2D discrete metallo-supramolecules.
The characterization of these hexagonal grids with disordered domains is extremely challenging using conventional approaches, e.g., NMR, X-ray diffraction, and mass spectrometry. Low-temperature scanning tunneling microscopy (UHV-LT-STM) was used to characterize the folded intermediates and self-assembled architectures at the atomic level. Furthermore, with scanning tunneling spectroscopy (STS), we were able to unambiguously identify 14 grid isomers that provide for unique distributions of metal ions for the first time. More broadly, we believe that the present demonstration of the bottom-up preparation of 20-nm-sized molecules with precisely controlled shapes and structures will advance our understanding of the design principles governing the preparation of discrete 2D self-assembled constructs and allow access to meso-sized materials with as-yet unprecedented functions and properties.
For details, please read our paper on Nature Chemistry (Nat. Chem., 2020, 12, 468), which was highlighted by Prof. Y. Wang on Nature Chemistry entitled Mesoscale Coordination Constructs (Nat. Chem., 2020, 12, 431). This study was also reported by entitled C&EN Superhuge Supramolecule (C&EN 2020, 98(15), 9) and selected as Molecules of Year 2020 (C&EN 2020, 98(48), 40).
The growing impact of the mechanical bond on polymer chemistry and materials science has already attracted a tremendous amount of attention in the design and synthesis of functional materials with remarkable properties. Among these materials, polyrotaxanes with multiple rings threaded onto polymer chains are, in particular, one of the most-widely explored synthetic targets for slide-ring gels that display unique physical characteristics and materials properties because of their mobile cross-linking components. In the collaboration with Sir Fraser Stoddart, we used mass spectrometry to obtain the most straightforward evidence for the formation for polyrotaxanes as well as a working mechanism of a polyrotaxane synthesizer. For details, please read our collaborative paper on Science (Science, 2020, 368, 1247). Such a polyrotaxane synthesizer was selected as Molecules of Year 2020 (C&EN 2020, 98(48), 40).
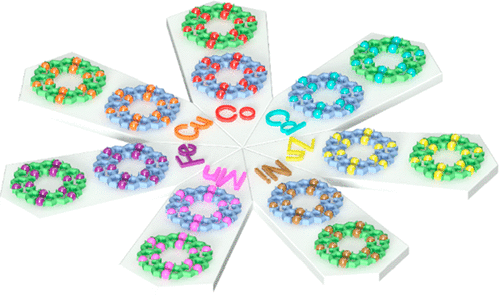
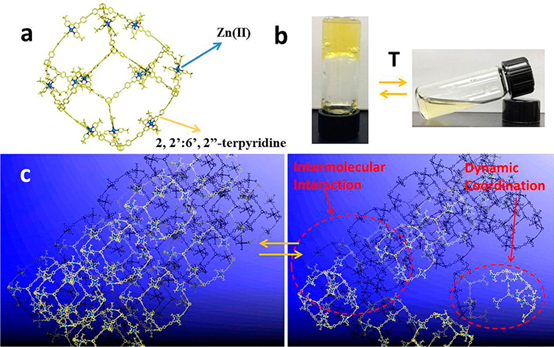
In terpyridine(tpy)-based supramolecular chemistry, the metal ions used to construct large discrete structures through direct self-assembly were mainly limited to three metal ions with highly reversible coordination, viz., Cd(II), Zn(II), and Fe(II). In this study, we significantly broaden the spectrum of metal ions with seven divalent transition metal ions M(II) (M = Mn, Fe, Co, Ni, Cu, Zn, Cd) to assemble a series of supramolecular fractals. In particular, Mn(II), Co(II), Ni(II), and Cu(II) were reported for the first time to form such large and discrete structures with ⟨tpy-M-tpy⟩ connectivity. Instead of building new structure, we studied the structural stabilities of those supramolecules in the gas phase and the kinetics of the ligand exchange process in solution using mass spectrometry. Such a fundamental study gave the relative order of structural stability in the gas phase and revealed the inertness of coordination in solution depending on the metal ions. Those results would guide the future study in tpy-based supramolecular chemistry in terms of self-assembly, characterization, property, and application. See our recent paper on J. Am. Chem. Soc. (J. Am. Chem. Soc., 2020, 142, 1811).

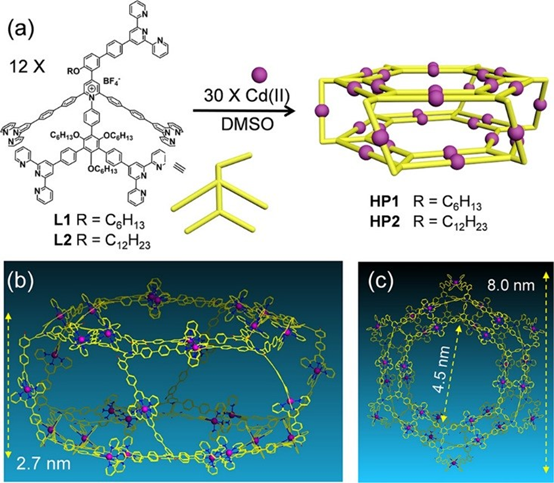
In the journey of exploring pyrylium salts and pyridinium salts chemistry, all the complex supramolecules we built were limited to 2D structures. Heng advanced the chemistry into 3D structure through using pentatopic terpyridine ligands to assemble two giant supramolecular hexagonal prisms, MW 42608 and 43569 Da (J. Am. Chem. Soc., 2019, 141, 16108). Within the prisms, two double-rimmed Kandinsky Circles serve as the base surfaces as well as the templates for assisting the self-sorting during the self-assembly. These supramolecular prisms show good antimicrobial activities against Gram-positive pathogen methicillin-resistant Staphylococcus aureus (MRSA) and Bacillus subtilis (B. subtilis).
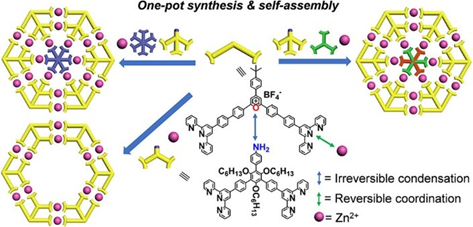
In the past several years, we always synthesized multi-armed organic building blocks for the self-assembly of complex structures with high density of coordination site (DOCS). Many group members complained about the multistep synthesis and tedious separation. Thanks to Heng, we are able to combine synthesis and self-assembly in one pot to construct supramolecular Kandinsky Circles and spiderwebs (J. Am. Chem. Soc., 2019, 141, 13187).

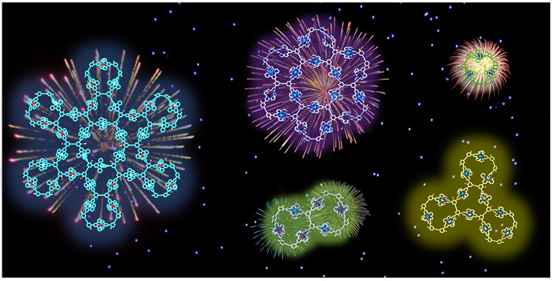
Most of the metallo-supramolecules were constructed with simple building blocks through coordination-driven self-assembly. Even now, using simple building blocks to construct complex structures with similar complexity as nature does is still the mainstream in self-assembly field. Inspired by the complex structure of protein, we attempted to introduce specific sequence into the building blocks of metallo-supramolecules. Please find our generations 1-5 supramolecular fractals with sequence-specific building block (Nat. Commun., 2018, 9, 4575) as well as the story Behind the Paper. Meanwhile, we obtained another series of supramolecular fractal with similar design principle with cover article published on J. Am. Chem. Soc. (J. Am. Chem. Soc., 2018, 140, 14087).


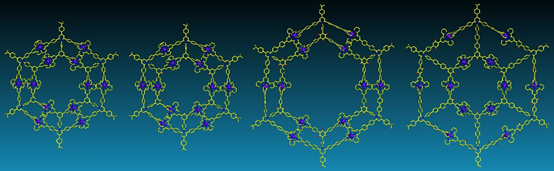
Nested concentric structures widely exist in nature and designed systems with circles, polygons, polyhedra, and spheres sharing the same center or axis. In art field, concentric rings are known as Kandinsky circles named after Wassily Kandinsky, a pioneer in abstract art, because of his prominent and profound painting color study-squares with concentric circles. We started Kandinsky Circles project in early 2013. After three years efforts, we assembled four G2 concentric hexagons (J. Am. Chem. Soc., 2016, 138, 9258) however, were unable to reach higher generation because of the challenging synthesis of multitopic terpyridine ligand.
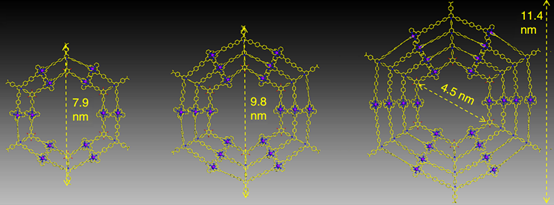
With another two years, we eventually found a solution with consecutive condensation between precursors with primary amines and pyrylium salts. Such modular synthetic approach allows us to prepare multi-armed terpyridine ligand, e.g., octatopic one. Then, three generations (G2-G4) of giant Kandinsky circles were assembled with molecular weight 17,964, 27,713 and 38,352 Da, respectively. Furthermore, such Kandinsky Circles displayed high antimicrobial activity against Gram-positive pathogen methicillin-resistant Staphylococcus aureus (MRSA) through formation of transmembrane channels. Our endeavors will shed light into both antibiotics and supramolecular chemistry field and pave a new avenue into the development and application of antimicrobial agents (Nat. Commun., 2018, 9, 1815).
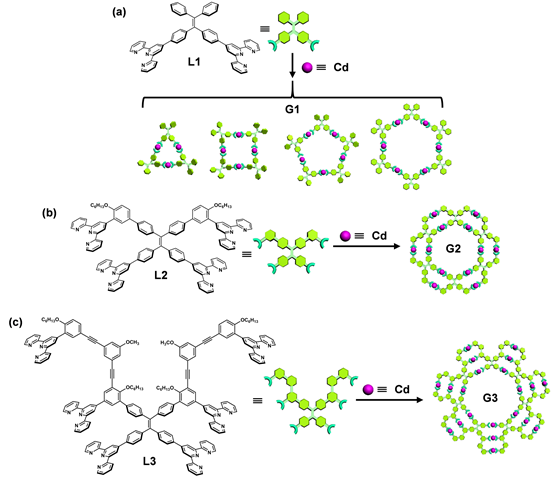
With the goal of increasing the restriction of intramolecular rotation (RIR) and structural complexity of supramolecules, we designed and synthesized L1-L3 by introducing multiple TPY groups onto tetraphenylethylene (TPE) core. The self-assembly of three generations of giant supramolecules G1-G3 with rosettes-like structures were further introducing additional restriction of intramolecular rotation and immobilize fluorophores. Such supramolecules display tunable emissive properties with respect to different generations, particularly, pure white-light emission. The single-component white light emitter is expected to exhibit superior performance improved stability, good reproducibility, and simple device fabrication procedure compared to those multi-component emitters. The team eventually named those structures as supramolecular rosettes, and hopefully, they can give out a bright light in both supramolecular chemistry and emissive materials community to inspire us to seek more complicated structures with tunable properties. The team was also invited by Nature Communications to write a story Behind the Paper (Nat. Commun., 2018, 9, 567).

After many years of attempts, we eventually made giant and rigid metallo-supramolecular snowflakes. This is also the first time we were able to combine three terpyridine ligands (multi-components) into one self-assembly system through step-wise approach. In the mixture of two-preassembled snowflakes, we even observed slow dynamic ligand exchange to form many hybrid snowflakes, indicating the great structural tolerance of these snowflakes. For many years, we wanted to publish this paper around Christmas; now it was published in May, Florida, where we have endless summer. Please see our recent paper on J. Am. Chem. Soc. (J. Am. Chem. Soc., 2017, 139, 8174).

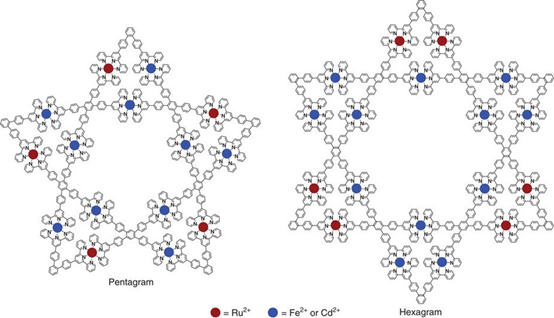
In the collaboration with Prof. Pingshan Wang, we successfully assembled supramolecular hexagram and pentagram using step-wise strategy. We originally named them as Star-of-David (hexagram) and Star-of-Texas (pentagram). Yes, the Star-of-Texas always let us recall Texas, the Lone Star State. Please find our recent publication on Nat. Commun. (Nat. Commun., 2017, 8, 15476).
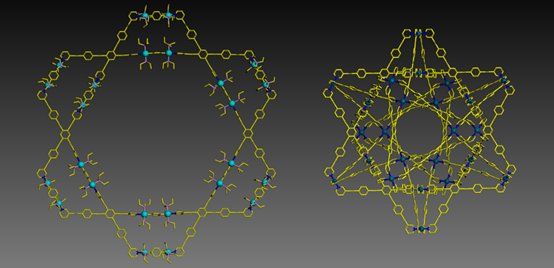
Meanwhile, we also advanced the supramolecular chemistry of Star-of-David from terpyridine-based self-assembly into pyridine-based self-assembly. Instead of using step-wise strategy, we redesigned the ligand and were able to directly assemble 2D Star-of-David with Pt(II)-component in high yield. With the goal of increasing the complexity and stability of supramolecules, for the first time, we obtained a 3D version of Star-of-David, which has four 2D Star-of-David as cross sections. For details, see our recent publication on Angew. Chem. Int. Ed. (Angew. Chem. Int. Ed., 2017, 56, 5258).
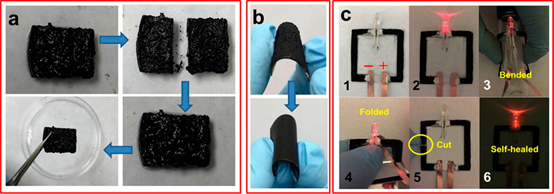
In the collaboration with Yu research group at University of Texas at Austin, we developed a hybrid material based on self-assembled supramolecular gel and nanostructured polypyrrole. This hybrid system synergizes the dynamic assembly/disassembly nature of metal-ligand supramolecular cubes and the conductive nanostructure of polypyrrole hydrogel and exhibits features of high conductivity (12 S m-1), appealing mechanical and electrical self-healing property without any external stimuli, and enhanced mechanical strength and flexibility (Nano Lett., 2015, 15, 6276).
Directed by increasing the density of coordination sites (DOCS) to increase the stability of assemblies, discrete 2D ring-in-rings and 3D sphere-in-sphere were designed and self-assembled by one tetratopic pyridyl-based ligand with 180° diplatinum(II) acceptors and naked Pd(II), respectively. The high DOCS resulted by multitopic ligand provided more geometric constraints to form discrete structures with high stability and complexity. Such sophisticated 2D and 3D structures with multiple subunits are reminiscent to protein tertiary structures, which contain different functional domains. If we introduce different functionality at different subunits, these sophisticated 2D and 3D architectures may perform as proteins with different functional domains, which can never be achieved in regular 2D macrocycles or 3D polyhedrons. We also proposed a quantitative definition of DOCS for 2D and 3D metallo-supramolecular structures (J. Am. Chem. Soc., 2015, 137, 1556).
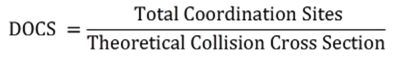
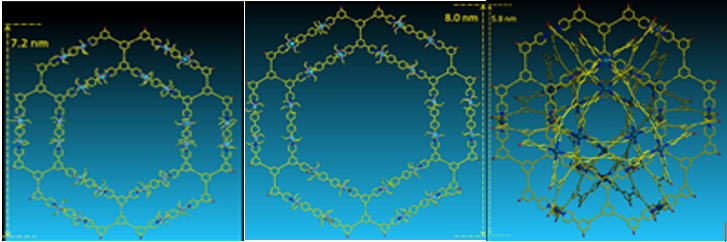
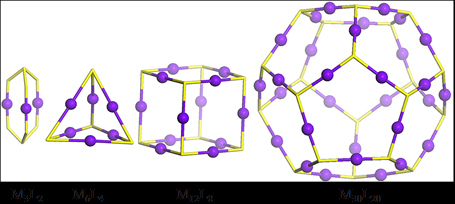
Using a series of tritopic 2,2’:6’,2”-terpyridine (tpy) ligands constructed on adamantane, three discrete 3D metallo-supramolecular architectures were assembled, i.e., trigonal bipyramidal, tetrahedron, and cube. The angles of the linkers between adamantane and tpy head play a critical role in guiding the assembled structures, which have the general formula of M3nL2n, where M denotes metal ion and L denotes ligand. And the self-sorting behavior is solely based on the angles precoded within the arm of tritopic ligands. More importantly, these cages may become an excellent system to study host−guest interaction due to their distinct cavity and face window sizes (J. Am. Chem. Soc., 2014, 136, 10499).
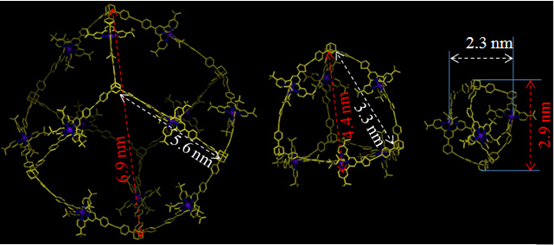
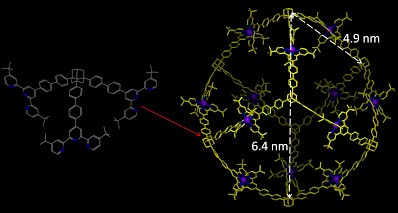
Conventional self-assembly of macrocycles that uses ditopic 2,2’:6’,2”-terpyridine (tpy) building blocks with a 120° angle between two ligating moieties generally produces a mixture of multiple macrocycles instead of a single hexagon. Precise control over the self-assembly of metallomacrocycles toward discrete structures is still one of the ultimate goals and challenges in the field of supramolecular chemistry. We reason that a system with a high density of coordination sites (DOCS) can provide more geometric constraints to form discrete and thermodynamically stable structures. Based on the principle to increase DOCS, we designed and assembled two fractal architectures by simple recursion of small hexagons around the center hexagon (J. Am. Chem. Soc., 2014, 136, 6664).
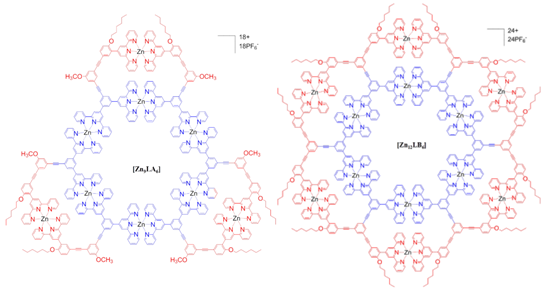
The construction of 3D architectures using 2,2’:6’,2”-terpyridine (tpy) was very challenging, mainly attributed to the following two reasons: (1) the fixed geometry of tpy-M(II)-tpy connectivity (i.e., 180°) and (2) the synthesis of appropriate organic ligands as directing units in the corners. Therefore, the self-assembly based on tpy was focused on supramolecular polymers, macrocycles, grid array, and 2D architectures. In this study, we utilized tpy-based organic ligands built on adamantane as directing units in the vertices to construct giant 3-D supramolecular cubes possessing highly symmetric structure with metal ions on edges (Chem. Sci., 2014, 5, 1221).
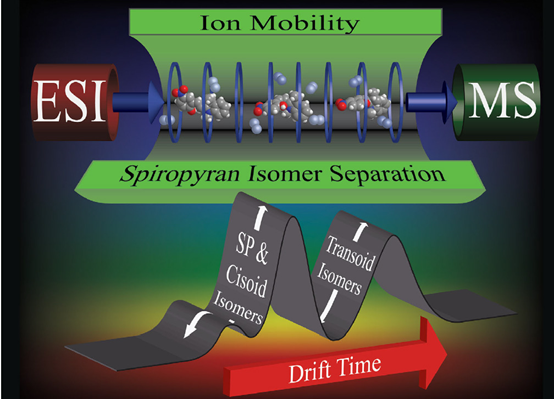
In collaboration with Brittain group, we used electrospray ionization (ESI) ion mobility-mass spectrometry (IM-MS) to study spiropyran photoisomerization, in which three major conformers were identified. This suggests that dynamic behaviour is temporarily “frozen” when the molecules are transferred from solution (thermodynamically controlled) to gas phase (kinetically controlled). The combination of ESI with IM-MS provides additional shapes and sizes information on the structure-dynamics of this important photochromic system. This study was featured as back cover (Chem. Commun., 2014, 50, 3424).

|
Visitors
|